Bisphosphonate Cost Comparison Calculator
Compare the annual and total costs of bisphosphonate treatments for bone metastases. Based on the article data showing cost differences between oral and intravenous options.
Cost Comparison Results
Alendronate (Oral): $100/year
Annual cost:
Total cost:
Zoledronic Acid (IV): $3,600/year
Annual cost:
Total cost:
Denosumab (Subcutaneous): $5,000/year
Annual cost:
Total cost:
Key Takeaways
- Alendronate is an oral bisphosphonate that can lower the risk of skeletal‑related events in patients with bone metastases.
- Clinical evidence shows it works best when combined with calcium and vitamin D supplementation.
- Kidney function, gastrointestinal tolerance, and drug interactions are the main safety concerns.
- Compared with intravenous agents like zoledronic acid or denosumab, alendronate is cheaper but slower to act.
- Choosing the right bisphosphonate depends on cancer type, disease stage, and patient preferences.
When a cancer spreads to the skeleton, the resulting pain, fractures, and hyper‑calcemia can be as devastating as the tumor itself. Doctors often turn to drugs that slow down bone breakdown, calling them bisphosphonates. Alendronate is a nitrogen‑containing bisphosphonate that you usually take as a weekly tablet. It works by binding to the bone surface and inhibiting osteoclast‑mediated resorption. Because it’s taken orally, it’s convenient for long‑term use, but it also raises questions about how well it helps cancer patients with bone metastases.
What Are Bone Metastases?
Bone metastases are cancer cells that have left their original organ and lodged in bone tissue. Common primaries include breast, prostate, lung, and kidney cancers. Once in bone, tumor cells release factors that stimulate osteoclasts, leading to rapid bone loss. The cascade creates three main problems:
- Pathologic fractures that can cripple a patient.
- Severe pain requiring high‑dose opioids.
- Elevated calcium levels (hyper‑calcemia) that threaten heart and kidney function.
These complications are grouped under the term skeletal‑related events (SREs). Preventing SREs is a primary goal of bone‑targeted therapy.
How Alendronate Works in the Context of Cancer
Alendronate’s mechanism is simple: it binds to hydroxyapatite crystals in bone and is taken up by osteoclasts during resorption. Inside the cell, it blocks the enzyme farnesyl pyrophosphate synthase, which is crucial for osteoclast function. The result is reduced bone turnover and a more stable skeletal matrix.
In cancer patients, the drug’s impact is twofold:
- Direct effect: Slows the resorption that tumor cells trigger, thereby reducing the space for new tumor growth.
- Indirect effect: Lowers calcium release into the bloodstream, helping manage hyper‑calcemia.
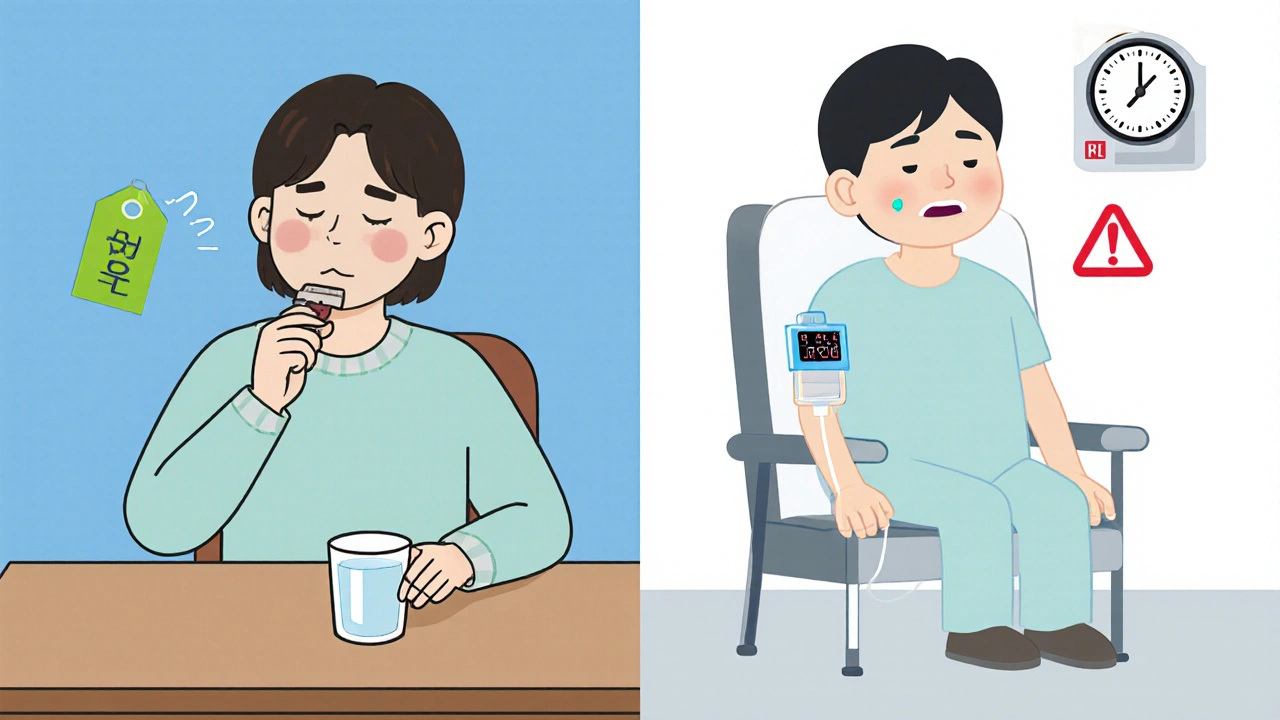
Because alendronate is taken orally once a week, adherence can be higher than with weekly infusion schedules, provided patients can tolerate the medication.
Evidence From Clinical Trials
Several randomized controlled trials (RCTs) have evaluated alendronate in cancer‑related bone disease. The most cited study is the ECOG‑5592 trial, which enrolled 1,500 patients with breast cancer bone metastases. Participants received either 70mg weekly alendronate plus calcium/vitaminD or placebo. After 24months, the alendronate group showed a 30% reduction in SREs (fracture, spinal cord compression, or need for radiation/surgery to bone).
Another key trial focused on prostate cancer patients. In a cohort of 800 men, alendronate lowered the incidence of pathological fractures from 12% to 7% over two years. Notably, pain scores improved by an average of 1.5 points on a 10‑point visual analog scale.
While the data is encouraging, it’s not as robust as the evidence for intravenous agents. Intravenous zoledronic acid and denosumab have demonstrated faster reductions in bone turnover markers and fewer SREs in the first six months. Alendronate’s advantage lies in cost and convenience, making it an attractive option when patients have good gastrointestinal health and stable renal function.
Safety Profile and Monitoring
Safety concerns for alendronate revolve around three areas:
| Issue | Typical Management |
|---|---|
| Gastro‑intestinal irritation | Take with a full glass of water, stay upright for 30minutes, avoid mineral supplements at the same time. |
| Renal impairment | Check serum creatinine; avoid if eGFR <30mL/min/1.73m². |
| Osteonecrosis of the jaw (ONJ) | Dental exam before starting therapy; maintain good oral hygiene. |
| Hypocalcemia | Supplement calcium (1,000mg) and vitaminD (800IU) daily. |
Oral bisphosphonates like alendronate have a lower reported incidence of ONJ than IV agents, but the risk is not zero. The FDA (U.S. Food and Drug Administration) currently requires a black‑box warning for both oral and IV bisphosphonates concerning ONJ and atypical femoral fractures.
When to Choose Alendronate Over IV Alternatives
Deciding between alendronate and agents like zoledronic acid or denosumab depends on several practical factors:
- Renal function: Alendronate is safe down to eGFR≈30mL/min, while zoledronic acid needs eGFR>30mL/min to avoid accumulation.
- Patient preference: Some patients dislike clinic infusions or have limited access to oncology infusion centers.
- Cost considerations: Oral alendronate costs roughly $0.20 per tablet in the U.S., whereas a single dose of zoledronic acid can exceed $300.
- Speed of action: For rapid control of bone pain, IV agents act within days; alendronate may take weeks to show maximal effect.
In practice, many oncologists start patients on a potent IV agent to gain quick control, then switch to alendronate for maintenance, especially if the disease is stable and the patient prefers oral therapy.
Practical Tips for Prescribing Alendronate to Cancer Patients
- Screen for contraindications: Confirm no esophageal stricture, active gastric ulcer, or severe renal impairment.
- Arrange baseline labs: Calcium, vitaminD, creatinine, and alkaline phosphatase.
- Educate on administration: Take the tablet with 8‑oz water, stay upright, and avoid food for 30minutes.
- Supplement wisely: Provide calcium (1,000mg) and vitaminD (800-1,000IU) daily unless contraindicated.
- Schedule monitoring: Re‑check calcium and kidney function every 3months; bone turnover markers (e.g., CTX) every 6months if available.
- Watch for side effects: Promptly address any new dysphagia, abdominal pain, or atypical thigh pain.
- Dental check‑up: A dental evaluation before starting therapy reduces ONJ risk.
Following these steps can help you get the most benefit from alendronate while keeping risks low.
Comparison of Oral vs. IV Bisphosphonates for Bone Metastases
| Feature | Alendronate (oral) | Zoledronic Acid (IV) | Denosumab (sub‑Q) |
|---|---|---|---|
| Administration | Weekly tablet | 3‑monthly infusion | Monthly injection |
| Onset of pain control | 4-6 weeks | Days to 2 weeks | Days to 2 weeks |
| Renal safety threshold | eGFR≥30mL/min | eGFR≥30mL/min | Not renal‑dependent |
| Risk of ONJ | Low (≈0.5%) | Moderate (≈1%) | Low‑moderate (≈0.8%) |
| Cost (US, per year) | ≈$100 | ≈$3,600 | ≈$5,000 |
| Typical indication | Bone metastases, osteoporosis | Bone metastases, hyper‑calcemia | Bone metastases, prevention of SREs |
The table shows why many clinics keep alendronate on the formulary: it’s cheap and easy. However, when rapid SRE prevention is critical, the IV options win despite higher price tags.
Future Directions and Ongoing Research
Researchers are testing higher‑dose weekly alendronate regimens to see if they can close the efficacy gap with IV agents. Early phase II data suggests a 40% reduction in bone turnover markers without extra GI toxicity. Additionally, combination studies pairing alendronate with newer bone‑targeted antibodies (e.g., romosozumab) aim to both inhibit resorption and stimulate formation, potentially offering a dual‑action approach.
The FDA is also reviewing labeling changes to simplify dosing instructions for cancer patients, which could improve adherence further.
Bottom Line for Clinicians and Patients
If you’re caring for a cancer patient with bone metastases and you need a cost‑effective, patient‑friendly option, alendronate is a solid choice-provided the patient can swallow tablets and has adequate kidney function. Combine it with calcium/vitaminD, monitor labs, and keep an eye on GI comfort. For those who need fast pain relief or have severe renal impairment, shift to an IV bisphosphonate or denosumab. The key is to tailor therapy to the individual’s medical profile and lifestyle.
Frequently Asked Questions
Can alendronate prevent new bone metastases?
No. Alendronate does not stop cancer cells from spreading to bone. It only helps reduce bone loss and the risk of skeletal‑related events once metastases are present.
How long should a patient stay on alendronate?
Most oncologists continue therapy for as long as the disease is active and the patient tolerates the drug, often for 2‑5years. If the patient achieves a disease‑free interval, a break can be considered under specialist guidance.
Is it safe to take alendronate with chemotherapy?
Generally yes, but timing matters. Give the chemotherapy infusion at least a few hours apart from the alendronate dose to avoid overlapping nausea or GI irritation.
What should I do if I develop esophageal pain?
Stop the tablet immediately and contact your oncologist. An endoscopic evaluation may be needed, and the doctor might switch you to an IV bisphosphonate.
Do I need a dental check‑up before starting alendronate?
A baseline dental exam is recommended to reduce the risk of osteonecrosis of the jaw, especially if you have a history of dental extractions or poor oral hygiene.

Rebecca Mitchell
October 17, 2025 AT 12:46If you’re not checking kidney function before giving alendronate you’re practically gambling with your patients' lives.
Cindy Thomas
October 21, 2025 AT 00:06While the data you present sounds impressive, the reality is alendronate’s modest effect often gets overstated :) In large‑scale trials the absolute reduction in skeletal‑related events hovers around ten percent, which, although statistically significant, may not translate into meaningful clinical benefit for every patient. Moreover, the requirement for strict calcium and vitamin D supplementation adds another layer of complexity that many clinicians overlook. You also ignore the fact that adherence rates drop sharply once patients experience even mild gastrointestinal discomfort. The comparison with IV agents should factor in the rapid pain control that alendronate simply cannot match. So before championing it as a cost‑saving miracle, consider the nuanced trade‑offs involved.
Kate Marr
October 24, 2025 AT 11:26🇺🇸 When you look at the price tag on alendronate versus the IV options, it’s clear why American patients love the oral route 😤 It slashes drug costs dramatically, letting insurers cover it with a modest copay. However, the convenience comes at the price of slower onset, which can be a deal‑breaker for those battling aggressive bone disease. Still, for many, the ability to avoid frequent clinic visits outweighs the delayed pain relief.
James Falcone
October 27, 2025 AT 21:46Yo, the cheap pill vibe is all good until your gut starts fighting back, then you’re back to the drip.
Frank Diaz
October 31, 2025 AT 09:06The discourse around alendronate often glosses over the metaphysical tension between patient autonomy and medical paternalism. By prescribing a tablet, the clinician ostensibly empowers the individual, yet the strict regimen subtly reinscribes control, dictating posture, timing, and adjunct supplementation. Such constraints betray an illusion of freedom, highlighting a deeper ethical paradox that is rarely interrogated in clinical guidelines. In evaluating efficacy, one must also weigh this philosophical cost against the biochemical gains.
Kevin Adams
November 3, 2025 AT 20:26Alendronate, though humble in appearance, carries a weight of clinical nuance that many overlook. Its oral administration offers a sense of autonomy rarely granted by intravenous regimens. Patients can swallow a tablet at home, sidestepping the logistical nightmare of infusion centers. Yet this convenience is a double‑edged sword, for adherence hinges on strict dietary rules that many find burdensome. The requirement to remain upright for half an hour after dosing, with a full glass of water, is a ritual that can be easily forgotten. When missed, esophageal irritation can cascade into ulceration, a complication that undermines the very purpose of therapy. Moreover, the onset of pain relief lags behind that of zoledronic acid, often taking six weeks to manifest measurable benefit. In the early months, patients may feel as though nothing is happening, prompting unnecessary switches to more aggressive agents. From a pharmacoeconomic perspective, alendronate shines like a modest beacon; its annual cost is a fraction of its IV counterparts. This affordability can translate into broader access, especially in underserved healthcare systems. However, cost should not eclipse safety, for renal function must be vigilantly monitored, with eGFR thresholds respected. The drug’s low renal toxicity profile is advantageous, yet dosing errors in patients with borderline function can still precipitate accumulation. In the realm of bone turnover markers, alendronate demonstrates steady, albeit slower, suppression compared to denosumab. Clinicians, therefore, face a delicate balancing act: weighing speed of action against long‑term sustainability and patient preference. Ultimately, the decision to prescribe alendronate should be anchored in a shared decision‑making process, where the patient’s lifestyle, comorbidities, and financial realities are given equal footing.
RJ Samuel
November 7, 2025 AT 07:46Well, that poetic ode to alendronate is a touch melodramatic, isn’t it? I’d argue the real drama lies in the bureaucratic hoops patients jump through to keep a simple pill on the shelves. While you wax lyrical about shared decision‑making, many are left navigating insurance labyrinths with no compass. So, the drama isn’t the drug; it’s the system that forces us to dramatise the mundane.
Miriam Rahel
November 10, 2025 AT 19:06Indeed, the systemic obstacles you mention are well‑documented in health economics literature, yet the pharmacologic profile of alendronate remains unequivocally favorable when stratified by renal function. A succinct appraisal of the cost‑benefit ratio would suffice, rather than an extended lamentation.
Mary Davies
November 14, 2025 AT 06:26Passive observation does not replace proactive monitoring.