Drug Withdrawal Time Calculator
How Drug Withdrawals Work
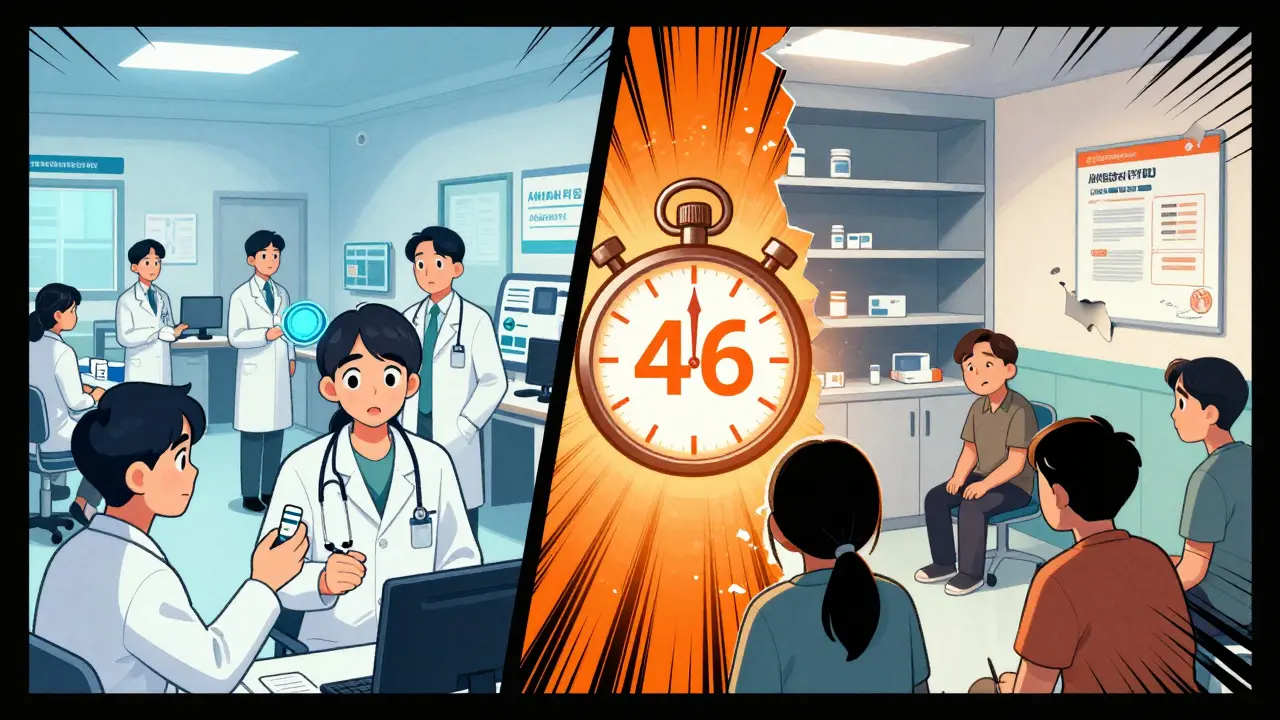
Before 2023, drug withdrawals took an average of 46 months (nearly 4 years) after evidence showed a drug was ineffective. The new process requires a decision within 180 days.
Between 2010-2020, 12.7% of drugs approved under accelerated pathways were withdrawn, with rates as high as 26% in oncology.
Result: Your calculated withdrawal timeframe depends on when the drug was approved.
Important Note: This calculator shows the maximum possible withdrawal time based on FDA data. Actual withdrawal times may vary based on specific circumstances.
When a drug is pulled from the market, it’s not just a corporate decision - it’s a safety alert. Millions of people rely on prescription medications every day, and when those drugs turn out to be unsafe or ineffective, the consequences can be serious. In recent years, high-profile cases like the withdrawal of Makena a drug approved in 2011 for preventing preterm birth, later withdrawn in 2022 after confirmatory trials showed it provided no benefit have exposed deep flaws in how the U.S. Food and Drug Administration (FDA) handles failed drugs. The system wasn’t built for speed. It was built for caution. But caution, when it takes years, can cost lives.
How Drugs Get Approved - and Why They Get Pulled
The FDA doesn’t approve drugs because they’re perfect. It approves them because, based on available data, their benefits outweigh their risks. For many drugs - especially those treating serious or life-threatening conditions - the agency uses a fast-track process called accelerated approval a pathway allowing drugs to reach patients based on surrogate endpoints, like tumor shrinkage, rather than long-term outcomes like survival. This means a drug can be on the market for years before its real-world effectiveness is fully confirmed. That’s where things go wrong. Between 2010 and 2020, about 12.7% of drugs approved under this fast-track system were eventually withdrawn. That’s one in eight. And in some cases, like a drug for small cell lung cancer, more than 41% of eligible patients received it - even after studies showed it didn’t work. The problem? The FDA had no legal power to act quickly. Sponsors could delay required follow-up studies indefinitely. The agency could only watch.The Old System: Years of Waiting
Before 2023, the FDA’s ability to remove a drug from the market was painfully slow. A 2023 study from the Penn LDI found that, on average, it took 46 months - nearly four years - to withdraw a drug after evidence showed it was ineffective. Compare that to the average approval time of about 12 months. That’s a 3.8-year gap between approval and removal. In the case of Makena, it took over 1,500 days to withdraw approval after the confirmatory trial failed. During that time, an estimated 150,000 patients received a drug that didn’t help them. Why so long? The process was bureaucratic. The FDA had to notify the drugmaker, allow time for appeals, open public comment periods, and sometimes convene advisory committees. There was no deadline. No urgency. And patients paid the price.The 2023 Change: A New Ruleset
The Consolidated Appropriations Act of 2023 changed everything. For the first time, the FDA gained real power to act fast. Under the new rules, the agency can now initiate a streamlined withdrawal process if:- The drug sponsor fails to conduct required post-approval studies
- The confirmatory study doesn’t verify the drug’s benefit
- Independent data proves the drug is unsafe or ineffective
- The company spreads false or misleading claims about the drug
Who Gets Affected? Patients, Doctors, Pharmacies
When a drug is withdrawn, it’s not just the manufacturer that feels the impact. Patients are caught off guard. Oncologists report that about 30% of their patients on accelerated approval drugs between 2015 and 2020 were prescribed therapies later pulled from the market. One breast cancer patient shared on a patient forum: “I was on [withdrawn drug] for 18 months before the trial failed. My doctor said it was standard care at the time.” Pharmacists struggle too. A 2022 survey found 63% had trouble interpreting the FDA’s Orange Book the official list of approved drug products with therapeutic equivalence evaluations, updated monthly to determine if a drug was withdrawn. That means patients might walk into a pharmacy and get a drug that’s no longer approved - because no one told the pharmacist. And doctors? They’re left scrambling. When a drug is pulled, practices have, on average, just 72 hours to find a replacement treatment for patients who were stable on it. That’s not enough time to review alternatives, check insurance coverage, or educate patients.Why This Matters Beyond Cancer Drugs
Most of the withdrawn drugs are in oncology - because that’s where accelerated approval is most common. But the same risks exist for drugs treating Alzheimer’s, rare diseases, and even chronic conditions like high cholesterol. The FDA has approved over 80 drugs through accelerated pathways since 2010. About 26% of those have been withdrawn. That’s not a small number. It’s a pattern. The new 2023 rules only apply to drugs approved after the law passed. So drugs like Makena, approved in 2011, are still stuck in the old system. That means more patients will keep getting drugs that don’t work - until the FDA finds a way to apply the new rules retroactively. For now, the system is still broken for many.
What’s Next? Real-World Data and Faster Decisions
The FDA is now piloting the use of real-world data from sources like Flatiron Health to monitor how drugs perform outside clinical trials. Instead of waiting years for a confirmatory study, the agency could spot problems faster by analyzing how thousands of patients respond in real time. The goal? Cut withdrawal time from 46 months to under 12. Commissioner Robert Califf says that’s achievable. But experts warn: speed without rigor could lead to premature withdrawals. A drug that looks ineffective in early data might still help a small group of patients. The challenge is balancing safety with access.What Patients Should Do
If you’re on a medication approved under accelerated approval - especially for cancer, rare diseases, or serious conditions - ask your doctor:- Is this drug approved under accelerated approval?
- Are there any pending confirmatory studies?
- Has the FDA issued any public notices about this drug?
Why This Isn’t Just About One Drug
Makena wasn’t the first drug withdrawn. It won’t be the last. But it became the symbol of a broken system. The FDA didn’t act because it didn’t have the power - not because it didn’t see the problem. The 2023 reforms were a wake-up call. They didn’t fix everything. But they gave the agency the tools to act. The real test isn’t whether the FDA can move faster. It’s whether it will - when the evidence is clear, and lives are on the line.What’s the difference between a drug recall and a withdrawal?
A recall happens when a drug is pulled from stores or pharmacies because of a manufacturing issue - like contamination or mislabeling. A withdrawal is when the FDA removes a drug because it’s unsafe or ineffective. Recalls are about quality. Withdrawals are about safety and performance.
Can a withdrawn drug still be prescribed?
Technically, yes - but it’s rare. Once a drug is withdrawn, manufacturers stop producing it, and pharmacies stop stocking it. Prescribers are strongly discouraged from using it, and insurance companies won’t cover it. Some patients may still have leftover pills, but continuing use is not recommended.
Why do drugs get approved if they might later be withdrawn?
For serious conditions with no other options, the FDA allows drugs to be approved based on early signs of benefit - like tumor shrinkage - while requiring confirmatory studies later. This gives patients access to potentially life-saving treatments faster. But if the later studies show no real benefit, the drug is withdrawn. It’s a gamble - and sometimes, patients lose.
How can I find out if my drug was withdrawn?
Check the FDA’s Orange Book online, search the Federal Register for withdrawal notices, or ask your pharmacist. The FDA also publishes monthly updates listing drugs withdrawn for safety or effectiveness. If your drug was approved after 2023, the FDA is required to notify prescribers directly - but many still miss the notice.
Are drug withdrawals common?
Not common overall, but they’re more frequent in certain areas. About 12.7% of drugs approved under accelerated pathways have been withdrawn since 2010. In oncology, that number jumps to 26%. Most withdrawals are due to lack of effectiveness, not safety issues.


John Sonnenberg
February 8, 2026 AT 02:15The FDA’s entire accelerated approval system is a house of cards built on hope and corporate lobbying. We’re talking about real people getting injected with drugs that don’t work-150,000 patients for Makena alone-and the agency sat on its hands for over four years. This isn’t bureaucracy. This is negligence dressed up as caution. And now they’re patting themselves on the back for cutting the timeline from 46 months to 180? That’s not progress. That’s damage control. The system still lets manufacturers run rings around regulators. No one’s accountable. No one’s punished. And patients? They’re the collateral.
Joshua Smith
February 9, 2026 AT 01:41I’ve been a pharmacist for 18 years, and this article hits way too close to home. We get the Orange Book updates, but they’re not always clear, and by the time we cross-reference everything, patients are already walking in with prescriptions for drugs that were pulled weeks ago. I’ve had to tell people their insurance won’t cover their med because it’s ‘no longer approved’-and they just stare at me like I’m the bad guy. It’s not our fault. But we’re the ones who have to explain it.
Elan Ricarte
February 10, 2026 AT 01:33Let’s be real-this whole system is a carnival ride designed to make Big Pharma rich and patients desperate. Accelerated approval? More like accelerated exploitation. They get the drug on the market, milk it for years, then quietly bury the follow-up studies. And when the FDA finally wakes up? They make a big show of ‘streamlined withdrawal’ like it’s some revolutionary win. Meanwhile, oncologists are still prescribing dead drugs because the damn system doesn’t auto-update their EHRs. No one’s auditing the audits. No one’s holding sponsors accountable. And we wonder why trust in medicine is collapsing.
THANGAVEL PARASAKTHI
February 10, 2026 AT 12:59India has similar issues but worse because we don’t even have a strong regulatory body like FDA. Many drugs approved here are pulled in US or EU but still sold here. Pharmacies don’t update lists. Doctors don’t check. Patients just keep taking them. I saw a man on a drug withdrawn in 2019-still getting it in 2024. No one told him. No one cared. This isn’t just an American problem. It’s global.
Ritteka Goyal
February 12, 2026 AT 09:37Why are we even surprised? The U.S. system is broken because it’s run by people who think profit matters more than life. Look at how fast they approved COVID vaccines-because panic. But for cancer drugs? Oh no, we need to wait 46 months. That’s not science. That’s hypocrisy. And now they want us to believe the 2023 change is a victory? It’s a band-aid on a hemorrhage. If they really cared, they’d use real-time data from every pharmacy, every hospital, every EHR-track outcomes live. Not wait for some corporate study to ‘fail.’
Jonah Mann
February 12, 2026 AT 20:23Just had to comment-this is why I always ask my oncologist if my med is on accelerated approval. I’ve been on two drugs that were later pulled. One was for melanoma. I was told it was ‘standard care.’ Turns out the confirmatory trial failed 8 months before I started. I was lucky-I switched in time. But my buddy? He kept taking it for a year after the withdrawal. His insurance denied it. He had to pay out of pocket. And the doctor? Never told him. That’s not malpractice. That’s systemic failure.
Scott Conner
February 13, 2026 AT 00:10So if a drug is withdrawn, can doctors still prescribe it? I thought that was illegal. But you said it’s ‘rare’-so it’s still happening. Who’s even checking? Are there penalties? Or is it just ‘we don’t really enforce this’? Also, how do patients even know if their drug is affected? The Orange Book is a nightmare to navigate. Why isn’t there a simple app or text alert? This feels like a 1990s system in a 2024 world.
Marie Fontaine
February 13, 2026 AT 21:21I’m a breast cancer survivor and I was on one of these withdrawn drugs. I didn’t know. My doctor said it was ‘the best option.’ I trusted her. I didn’t question it. When I found out later, I was devastated. Not because I didn’t get better-but because I spent 18 months on something that did nothing. And now I’m scared to try anything new. How do we fix trust? How do we make sure no one else goes through this? We need a public database. Simple. Real-time. For everyone.
Tatiana Barbosa
February 14, 2026 AT 22:31Real-world data is the only way forward. We’re not in the 1980s anymore. We have AI, we have wearables, we have EHRs that track every prescription, every lab result, every ER visit. Why are we still waiting for RCTs that take five years? The FDA should be mining data from Flatiron, Epic, and Kaiser like a hawk. If 30% of patients on a drug show no response within six months-pull it. No waiting. No hearings. No appeals. This isn’t about ‘rigor’-it’s about ethics. We owe patients faster answers. And if that means a few drugs get pulled prematurely? Better than letting thousands suffer on placebos with a price tag.
Ken Cooper
February 15, 2026 AT 02:59Wait-so the new rules only apply to drugs approved after 2023? That means Makena is still hanging around like a ghost? What’s the point of reform if it’s not retroactive? We’re talking about people still getting this drug right now. This isn’t a policy tweak. It’s a moral failure. And why isn’t the public screaming about this? Why aren’t patient advocacy groups organizing? This is the definition of systemic violence. And we’re just… shrugging? I’m furious.
Tricia O'Sullivan
February 15, 2026 AT 21:53While I appreciate the intent behind the 2023 reforms, I remain cautious about the balance between speed and due process. Regulatory agencies must not only be effective but also legitimate in the eyes of the public. A process that is too rapid risks undermining the very foundations of evidence-based medicine. There is value in deliberation-even when it is inconvenient. The challenge lies not in speed alone, but in ensuring that decisions are both timely and robustly substantiated.
Lyle Whyatt
February 17, 2026 AT 21:24I’ve been following this for years. The system isn’t broken-it’s been designed this way. Accelerated approval was never meant to be a shortcut. It was meant to be a loophole. A way for pharma to get drugs on the market before the FDA could say no. And now, with the new rules, they’re still not forced to pay a penalty. No fines. No jail time. No clawbacks. Just… ‘withdrawn.’ So the company loses a product. But the investors? They already cashed out. The executives? They got their bonuses. And the patients? They got nothing. This isn’t reform. It’s theater.
MANI V
February 19, 2026 AT 06:05People need to stop acting like the FDA is some saintly guardian. It’s a regulatory puppet. Controlled by lobbyists. Controlled by industry donations. Controlled by revolving door appointments. That’s why Makena stayed on the market for over a decade. That’s why 26% of oncology drugs get pulled. Because the system is rigged. And until we break the money chain-until we ban former pharma execs from running the FDA-nothing will change. This isn’t about science. It’s about power. And the powerless? They’re just collateral damage.