When talking about FDA approval, the official clearance by the U.S. Food and Drug Administration that a drug, device, or supplement meets safety and effectiveness standards. Also known as US FDA clearance, it guides everything from brand‑name pills to over‑the‑counter remedies.
One of the biggest side‑effects of FDA approval is that it creates a trusted pathway for generic medication, copies of brand‑name drugs that hit the market after the original patent expires. Generic makers still have to prove their product matches the original in dosage, strength, route, quality, performance characteristics, and intended use. Because of that, you can expect the same therapeutic effect at a lower price, but only when the FDA signs off.
Behind every approval sits a massive set of clinical trials, systematic studies that test safety, dosage, and efficacy in volunteers. The FDA looks at Phase I (safety), Phase II (effectiveness), and Phase III (large‑scale confirmation) data before granting a green light. If a trial shows serious side effects, the agency can delay or deny approval, which directly protects drug safety, the balance between therapeutic benefit and risk of harm.
First, approval means the product has passed a rigorous review. That review checks the chemistry, manufacturing process, labeling, and post‑market monitoring plans. Second, it gives pharmacies and online sellers a clear benchmark. When you see a medication listed as FDA‑approved, you know it has met a national standard, which cuts down the guesswork when comparing cheap generics, like Lipitor or Zyrtec, to their brand‑name counterparts.
Third, the FDA’s role ties directly into regulatory compliance. Companies that skip the approval process can face fines, product seizures, or lawsuits. Compliance also means keeping up with new safety alerts. For example, if a new study links a drug to rare side effects, the FDA can issue a label update or even pull the product. That ongoing oversight is why you’ll often see our articles mention the latest FDA alerts alongside drug comparisons.
So how does this impact your online shopping experience? When you search for cheap generic versions of popular meds, look for sites that verify the pharmacy’s license and display the FDA approval status. Our guides walk you through spotting legitimate pharmacies, checking batch numbers, and confirming that the product matches the FDA’s approved label.
Another practical tip: use the FDA’s “Drug Approval Database” to see when a drug was approved, what conditions it treats, and whether any post‑marketing studies are required. That database is free and searchable, and it’s a great way to double‑check before you click “buy.”
Lastly, keep an eye on emerging therapies that haven’t hit the market yet. Gene‑therapy treatments for hemophilia, for instance, are under FDA review and could become options soon. Knowing where a product stands in the approval pipeline helps you plan ahead and ask the right questions of your healthcare provider.
Below you’ll find a curated list of articles that dive deeper into specific drugs, their FDA status, safety considerations, and how to get them safely online. Whether you’re looking for a bisphosphonate for bone health, a fast‑acting ED medication, or the latest on antibiotic pricing, each post ties back to the core idea of FDA approval and what it means for you.
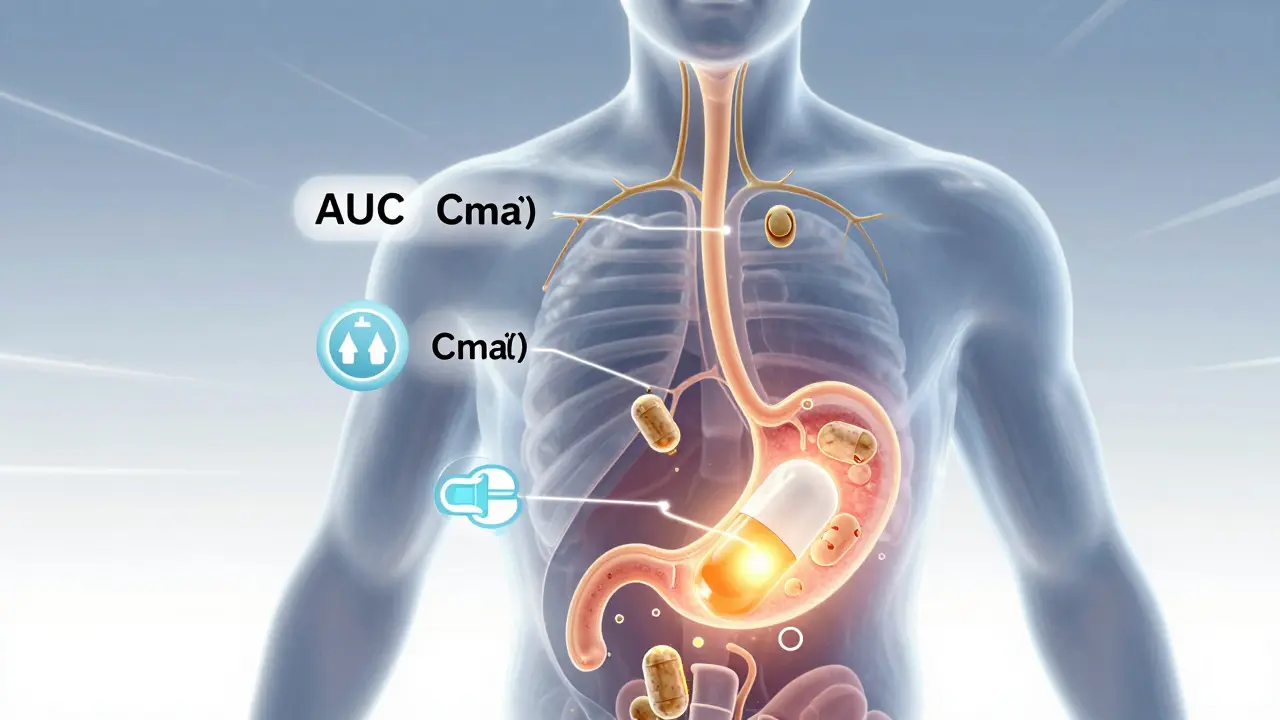
Bioavailability studies ensure generic drugs are absorbed the same way as brand-name versions. The FDA uses precise pharmacokinetic tests to confirm safety and effectiveness before approval.
View more
Explore flibanserin's role in treating low sexual desire, its controversial FDA approval, safety profile, gender bias in research, and the ongoing fight for female sexual equality.
View more