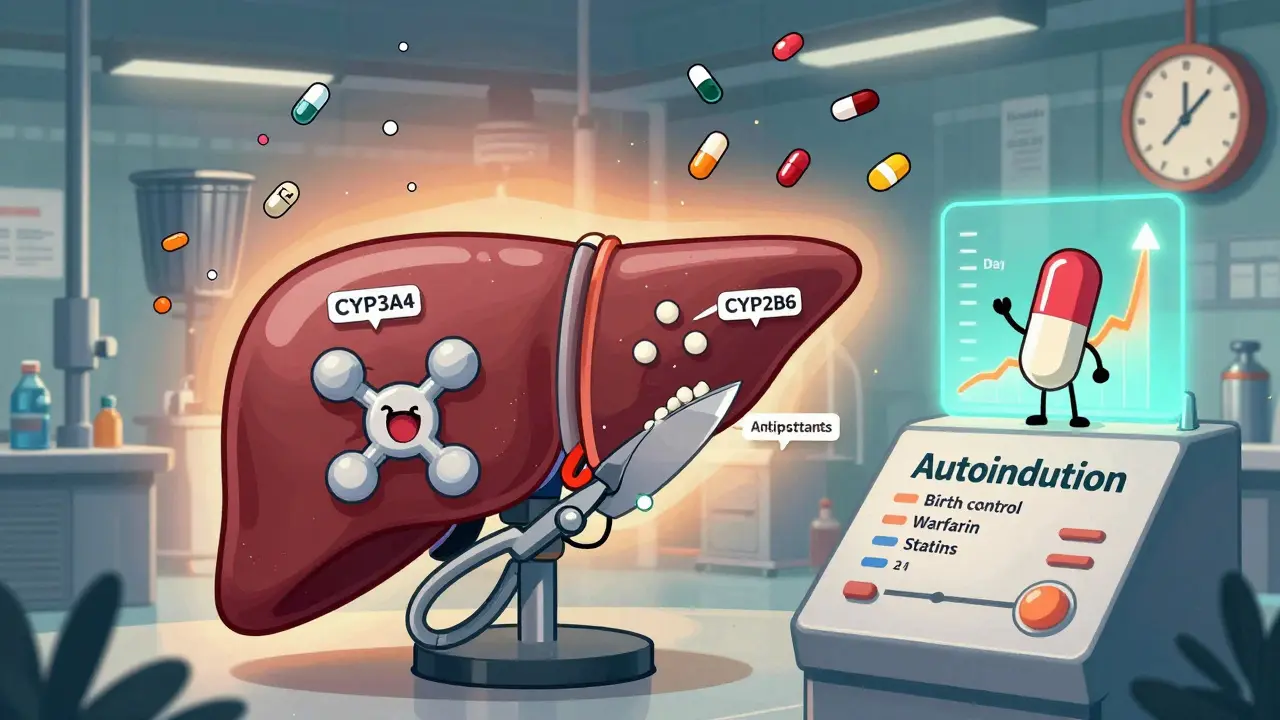
Carbamazepine is a powerful enzyme inducer that can reduce the effectiveness of birth control, blood thinners, antidepressants, and more. Learn how it affects other drugs, why autoinduction makes it tricky, and what to do to stay safe.
View more
Behind-the-counter medications like pseudoephedrine and Plan B require ID and pharmacist consultation but no prescription. Learn how they work, why they exist, and how to buy them without hassle.
View more
Most expired medications are still effective years after their labeled date, especially if stored properly. Science shows many pills retain potency for decades-except for a few high-risk drugs like insulin and EpiPens.
View more
Learn safe, practical ways to take your medicine if you have trouble swallowing pills. Discover alternatives to crushing tablets, proven techniques, and how to work with your care team to stay on track.
View more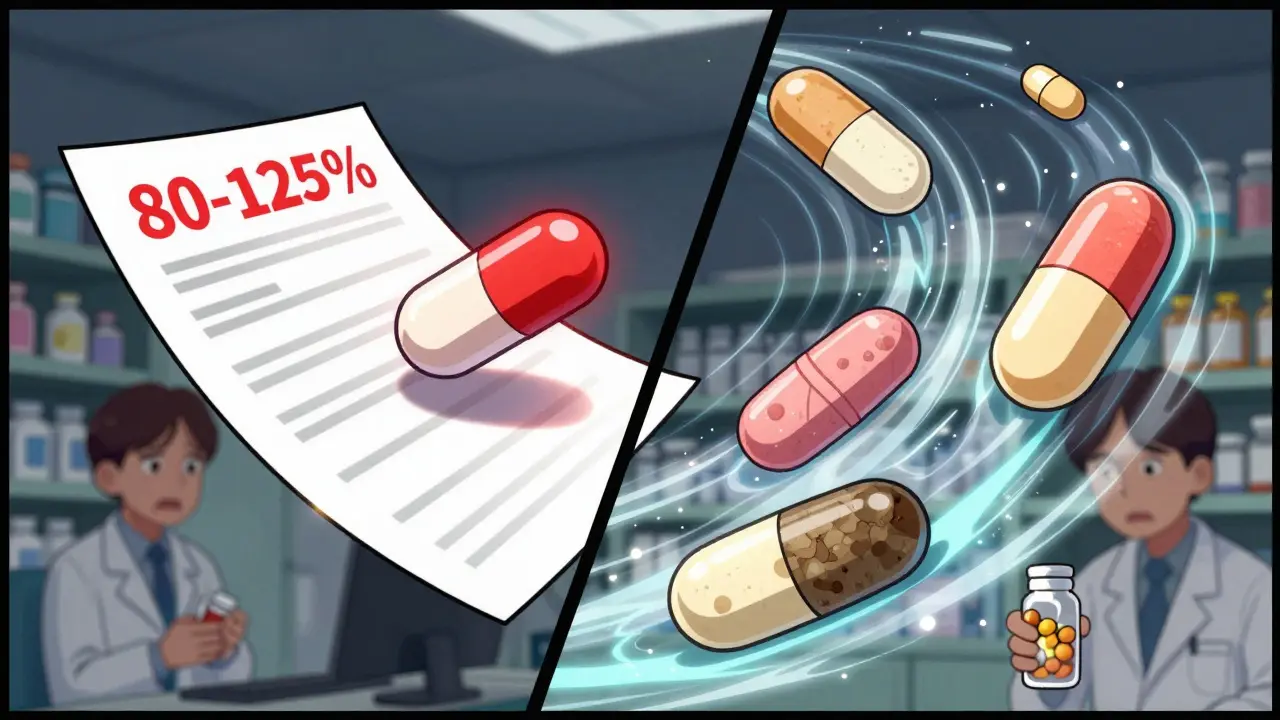
Batch-to-batch variability can skew bioequivalence results, leading to false approvals or rejections of generic drugs. Learn how regulators are updating standards to account for real-world manufacturing differences.
View more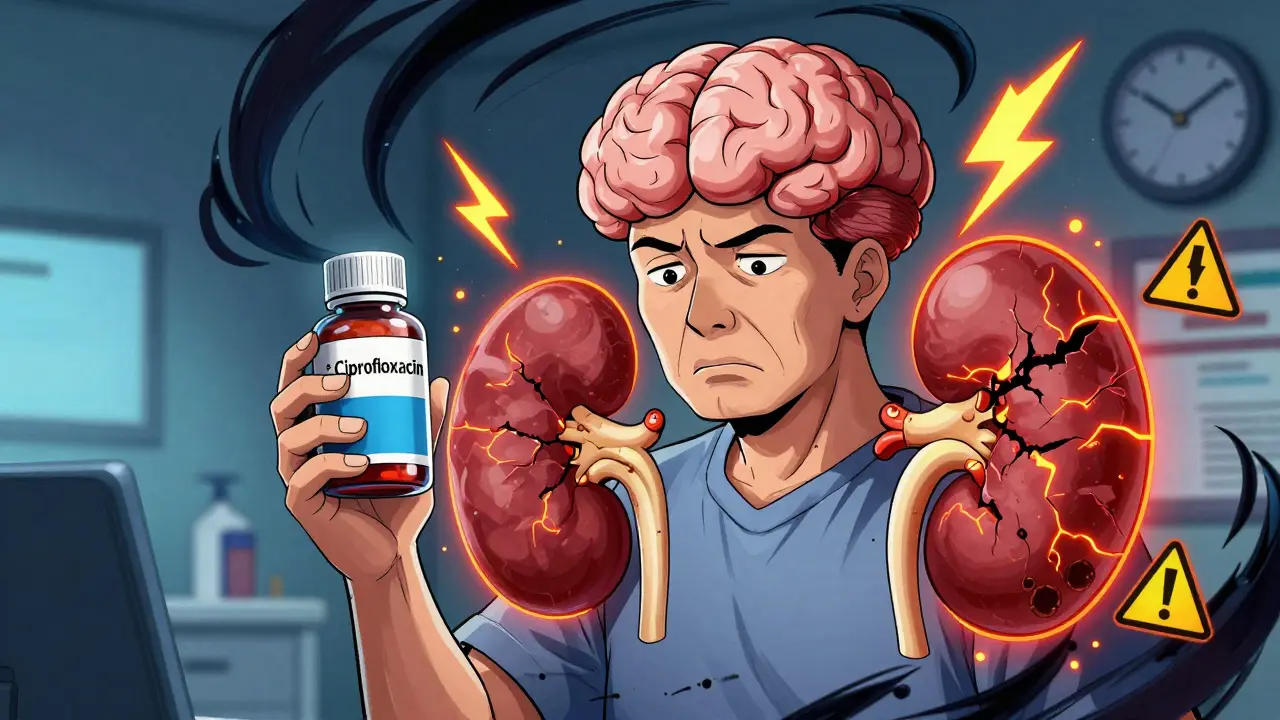
Combining fluoroquinolone antibiotics with NSAIDs increases the risk of severe kidney injury and neurological damage. Learn why this dangerous interaction happens and what safer alternatives exist.
View more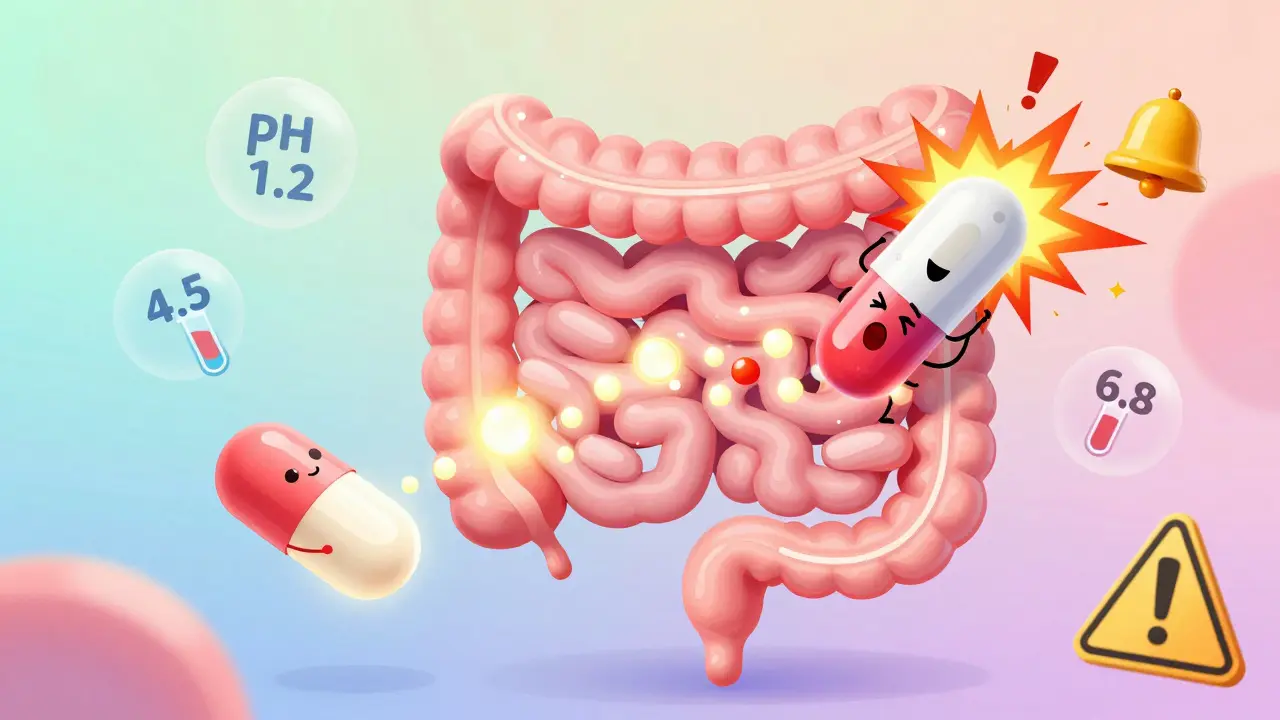
Modified-release formulations require special bioequivalence testing to ensure generic versions match the brand's release pattern. Learn the key rules, regulatory differences, and why many generics fail.
View more
Understanding how generic drugs degrade over time and why shelf life matters for safety. Learn the science behind stability testing, risks of expired medications, and how storage impacts effectiveness.
View more
When medications run out due to shortages, knowing your alternatives can prevent health risks. Learn how to find safe substitutes, work with your pharmacist, and navigate insurance changes during drug shortages in 2026.
View more
India supplies 20% of the world's generic medicines and over 60% of global vaccines. Learn how its low-cost, high-quality manufacturing powers global healthcare access.
View more